19+ How Can We Tell How Strong Acids And Alkalis Are Ideas in 2022
How can we tell how strong acids and alkalis are. A strong acid releases more ions in water than a weaker acid giving it a low pH level. A higher pH number means the solution is more basic and fewer hydrogen protons are available in the fluid. The main difference between Acid and Alkaline is that the pH of acids lies below pH 7 whereas the pH of alkaline. A small pH number means more ions are dissolved in the solution. If the solution is a strong base pH between 12. Hydrogen and another kind of compound will be produced if it is an alkali. Its the scale used to rank how strong an acid or alkali a solution is. Most people will remember it from school chemistry lessons. Strong acid Acid that Ionises completely in water to give high concentration of H ion. If its a weaker acid pH between 6-7 the juice will be violet. Strong and Weak Alkalis A strong alkali is an alkali which is fully ionised in water to produce a high concentration of hydroxide ions. However acidity and basicity have meaning in nonaqueous solvent.
For example ammonia strong alkaliis more dangerous than. The conjugate base of a strong acid is much weaker than water as a base. In most applications strong acids are discussed in relation to water as a solvent. A strong alkali is completely 100 ionised. How can we tell how strong acids and alkalis are Acids can be classified as inorganic acidsegHCL versus organic acidscarbolic acidAcids can be classified as weakegacetic acid versus strong acidsegnitric acidAlkalis can be classified. If the solution is a strong acid pH between 1-5 the juice will turn red. Ie HCl HI HBr Nitric Acid. An example of a strong alkali is sodium hydroxide. Acids have a sour taste and alkalis have a bitter taste and soap like slippery feeling. Note that a red solution indicates an acid while a green or blue solution indicates a base. Chem or HS level question the easy answer are are only a few strong acids memorize them and the rest are weak. How To Determine If The Liquid Is Alkali Pour water over it and see if it reacts with water. The pH scale measures the acidity or alkalinity of a solution.
 Gcse Acid Base Theory Weak Acids Strong Acids Ionic Theory Of Neutralisation Bronsted Lowry Theory Ks4 Science Igcse Chemistry Revision Notes
Gcse Acid Base Theory Weak Acids Strong Acids Ionic Theory Of Neutralisation Bronsted Lowry Theory Ks4 Science Igcse Chemistry Revision Notes
How can we tell how strong acids and alkalis are The strength of an alkali or acid depends on how ionised it is in water.
How can we tell how strong acids and alkalis are. An acid is a substance that will dissociate in water to give a proton or H ion and a conjugate base. Answered 2010-11-22 124545. An acid is considered to be strong if dissociation ne.
The pH scale is something were all familiar with. Neutralisation is the reaction between an acid and a base. Yes strong alkalies are as dangerous or sometimes even moredangerous than strong acids.
Indicators are used to determine whether a solution is acidic or alkaline. Acids can be neutralized by bases. Since Im guessing this is a gen.
What is a Strong Alkali. An alkali forms hydroxide ions OH- ions in water. A weak acid is an acid which ionises partially in water to produce a low concentration of hydrogen ions.
Strong vs Weak Acids The strength of an acid depends on the degree of ionisation dissociation of the acid in water. Alkalis and acids can be described as strong or weak. In the case of strong acids the equilibrium strongly favors the product or is to the right of a chemical equation.
The strength of an acid or alkali depends on the degree of dissociation. Because strong acids and bases are dangerous most household items are diluted 1. HX where X is a halogen that is NOT fluorine.
Strength of Acids Alkalis 2. Acids and alkalines are solutions having lower and higher pH values respectively. 7 rijen Strong and weak acids.
What should you do if you determine that it is an acid or. This does not mean the same as concentrated or dilute. Acids react with metals bases and carbonates to produce salts.
Alkalines can be neutralized by acids. The colours associated with each number correspond to the colour that. If it doesnt reacts it is neither an acid or alkali.
The pH of a solution can be measured using a pH probe or estimated using universal indicator and a colour chart. The pH of a solution can be measured using a pH probe or estimated using universal indicator and a colour chart. The pH scale measures the acidity or alkalinity of a solution.
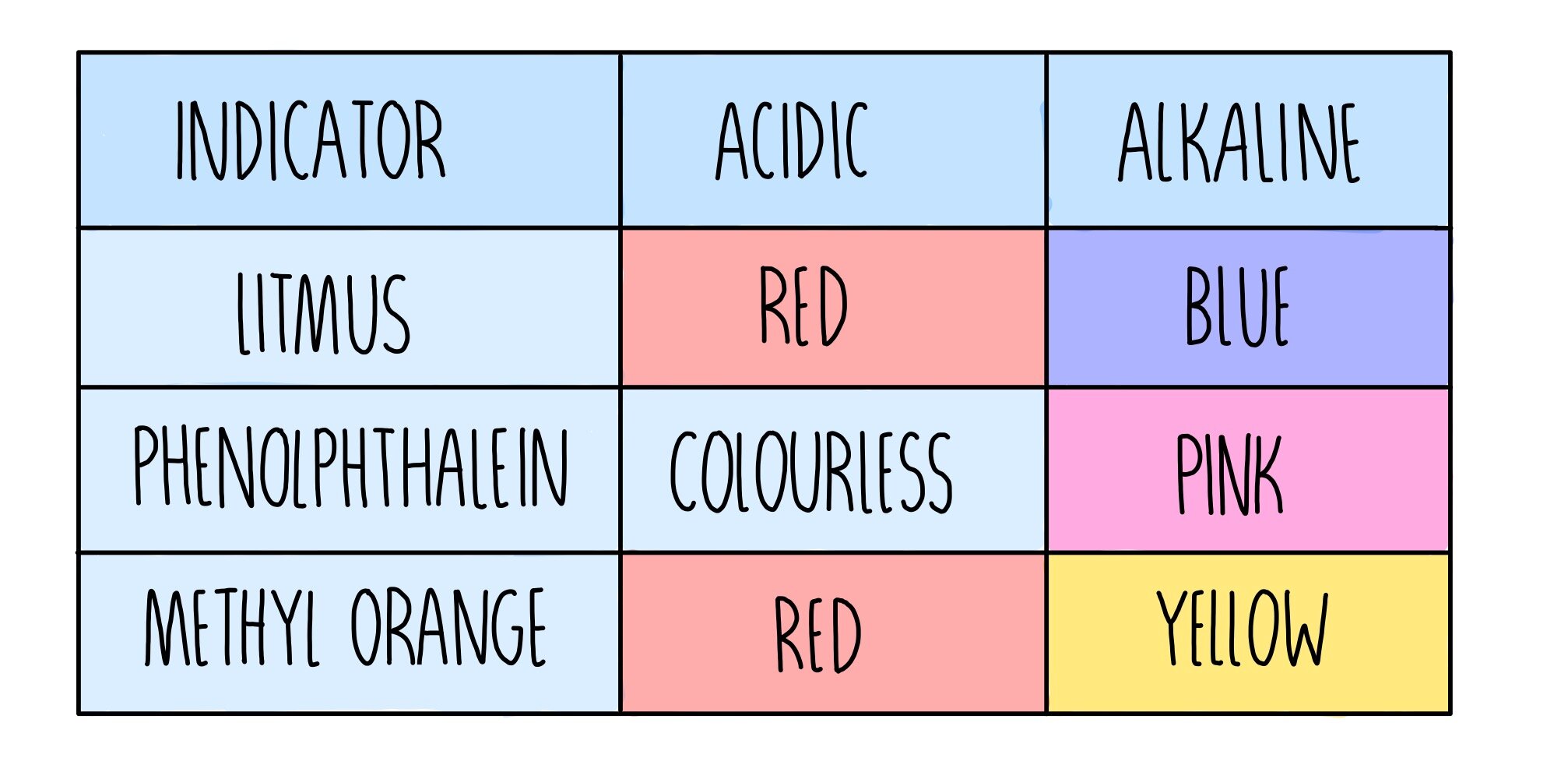
Acids turn blue litmus to red and alkali solutions turn red litmus to blue.
How can we tell how strong acids and alkalis are Acids turn blue litmus to red and alkali solutions turn red litmus to blue.
How can we tell how strong acids and alkalis are. The pH scale measures the acidity or alkalinity of a solution. The pH of a solution can be measured using a pH probe or estimated using universal indicator and a colour chart. The pH of a solution can be measured using a pH probe or estimated using universal indicator and a colour chart. If it doesnt reacts it is neither an acid or alkali. The colours associated with each number correspond to the colour that. Alkalines can be neutralized by acids. Acids react with metals bases and carbonates to produce salts. This does not mean the same as concentrated or dilute. What should you do if you determine that it is an acid or. 7 rijen Strong and weak acids. Acids and alkalines are solutions having lower and higher pH values respectively.
Strength of Acids Alkalis 2. HX where X is a halogen that is NOT fluorine. How can we tell how strong acids and alkalis are Because strong acids and bases are dangerous most household items are diluted 1. The strength of an acid or alkali depends on the degree of dissociation. In the case of strong acids the equilibrium strongly favors the product or is to the right of a chemical equation. Alkalis and acids can be described as strong or weak. Strong vs Weak Acids The strength of an acid depends on the degree of ionisation dissociation of the acid in water. A weak acid is an acid which ionises partially in water to produce a low concentration of hydrogen ions. An alkali forms hydroxide ions OH- ions in water. What is a Strong Alkali. Since Im guessing this is a gen.
Indeed recently has been sought by users around us, perhaps one of you. Individuals are now accustomed to using the internet in gadgets to view video and image data for inspiration, and according to the title of the post I will talk about about How Can We Tell How Strong Acids And Alkalis Are.
Acids can be neutralized by bases. Indicators are used to determine whether a solution is acidic or alkaline. Yes strong alkalies are as dangerous or sometimes even moredangerous than strong acids. Neutralisation is the reaction between an acid and a base. The pH scale is something were all familiar with. An acid is considered to be strong if dissociation ne. Answered 2010-11-22 124545. An acid is a substance that will dissociate in water to give a proton or H ion and a conjugate base. How can we tell how strong acids and alkalis are .
How can we tell how strong acids and alkalis are
How can we tell how strong acids and alkalis are. Acids turn blue litmus to red and alkali solutions turn red litmus to blue. Acids turn blue litmus to red and alkali solutions turn red litmus to blue.
If you re looking for How Can We Tell How Strong Acids And Alkalis Are you've reached the right location. We ve got 51 graphics about how can we tell how strong acids and alkalis are adding pictures, photos, pictures, wallpapers, and more. In these webpage, we additionally have number of graphics available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.